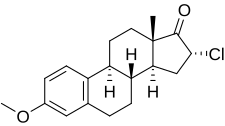
Clomestrone
 | |
| Clinical data | |
|---|---|
| Trade names | Arterolo, Atheran, Colesterel, Iposclerone, Liprotene, Persclerol |
| Other names | SC-8246; 16α-Chloroestrone 3-methyl ether; 16α-Chloro-3-methoxyestra-1,3,5(10)-trien-17-one |
| Routes of administration | By mouth |
| Drug class | Estrogen; Estrogen ether |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID |
|
| ChemSpider |
|
| UNII |
|
| CompTox Dashboard (EPA) |
|
| ECHA InfoCard | 100.021.669 |
| Chemical and physical data | |
| Formula | C19H23ClO2 |
| Molar mass | 318.84 g·mol−1 |
| 3D model (JSmol) |
|
| |
InChI
| |
Clomestrone (brand names Arterolo, Atheran, Colesterel, Iposclerone, Liprotene, Persclerol, others; former developmental code name SC-8246), also known as 16α-chloroestrone 3-methyl ether, is a synthetic, steroidal, weak estrogen derived from estrone and used as an anticholesterolemic agent in the treatment of atherosclerosis.[1][2] It is said to have beneficial effects on serum lipid profiles while producing minimal feminization, though some estrogenic side effects, including breast tenderness, loss of libido, and fatigue or avolition, were observed in most patients in clinical studies.[3][4] The drug is a close analogue of mytatrienediol, and the two estrogens have similar drug profiles.[5] Clomestrone was described in the literature in 1958 and introduced for medical use shortly thereafter.[1]
See also
References
- ^ a b J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 297–298. ISBN 978-1-4757-2085-3.
- ^ Gregory Pincus (22 October 2013). Hormones and Atherosclerosis: Proceedings of the Conference Held in Brighton, Utah, March 11-14, 1958. Elsevier Science. pp. 253–374. ISBN 978-1-4832-7064-7.
- ^ RIVIN AU (1959). "SC 8246, a new estrogen analog: lipoprotein effects with minimal feminization". Metab. Clin. Exp. 8: 704–8. PMID 14437693.
SC-8246 (16-alpha chlorestrone 3-methyl ether) was administered for periods of six to twelve months to 20 male survivors of acute myocardial infarction ranging in age from 30 to 63 years. A significant decrease in serum cholesterol concentration occurred in only 6 of 13 patients with an initial cholesterol level above 250 mg. per 100 ml., and there was no change in the other 7. Of 7 initial cholesterol levels below 250 mg. per 100 ml., no level decreased, 3 increased, and 4 were unchanged. In 9 of 11 patients with an initial alpha:beta lipoprotein ratio of less than 20 per cent, a significant increase occurred, but no change in the other 2. Among 9 subjects with a ratio initially above 20 per cent, a further increase occurred in 8 while taking the drug. This estrogen appeared to have an advantage in terms of lessening side-effects. Mild breast tenderness or gynecomastia occurred in 15 of the 17 patients with a "favorable" lipoprotein change. When the dosage was reduced to 5 mg. daily or every other day, the lipoprotein effect in 8 of them could be sustained while the breast changes disappeared. Libido disappeared from 2 patients and was diminished in 1 other. Other side-effects were nausea in 1 patient, loss of ambition in 5, and itching or dryness of the skin in 4.
- ^ WINSOR T, FISHER EK, PAYNE JH (1959). "A method for the study of peripheral arteriosclerosis". J Am Geriatr Soc. 7 (2): 167–74. doi:10.1111/j.1532-5415.1959.tb01062.x. PMID 13630690. S2CID 46048052.
- ^ Cancer Chemotherapy Abstracts. Information Resources Press. January 1960. p. 143.
- v
- t
- e
- #WHO-EM
- ‡Withdrawn from market
- Clinical trials:
- †Phase III
- §Never to phase III
- See also
- Estrogen receptor modulators
- Androgens and antiandrogens
- Progestogens and antiprogestogens
- List of estrogens
 | This article about a steroid is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e
 | This drug article relating to the genito-urinary system is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e
 | This drug article relating to the cardiovascular system is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e










