Mammalian protein found in Homo sapiens
| CEACAM1 |
|---|
 |
| Available structures |
|---|
| PDB | Ortholog search: P13688%20or%20M0R2K4 PDBe P13688,M0R2K4 RCSB |
|---|
| List of PDB id codes |
|---|
2GK2, 4QXW, 5DZL, 4WHD |
|
|
| Identifiers |
|---|
| Aliases | CEACAM1, BGP, BGP1, BGPI, carcinoembryonic antigen related cell adhesion molecule 1, CEA cell adhesion molecule 1 |
|---|
| External IDs | OMIM: 109770; MGI: 1347246; HomoloGene: 128630; GeneCards: CEACAM1; OMA:CEACAM1 - orthologs |
|---|
| Gene location (Human) |
|---|
 | | Chr. | Chromosome 19 (human)[1] |
|---|
| | Band | 19q13.2 | Start | 42,507,304 bp[1] |
|---|
| End | 42,561,234 bp[1] |
|---|
|
| Gene location (Mouse) |
|---|
 | | Chr. | Chromosome 7 (mouse)[2] |
|---|
| | Band | 7 A3|7 13.87 cM | Start | 25,215,467 bp[2] |
|---|
| End | 25,239,282 bp[2] |
|---|
|
| RNA expression pattern |
|---|
| Bgee | | Human | Mouse (ortholog) |
|---|
| Top expressed in | - rectum
- minor salivary glands
- jejunal mucosa
- duodenum
- bone marrow
- bone marrow cells
- appendix
- oral cavity
- parotid gland
- right lobe of liver
|
| | Top expressed in | - spermatid
- proximal tubule
- seminiferous tubule
- left colon
- secondary oocyte
- lens
- major salivary gland
- parotid gland
- duodenum
- intercostal muscle
|
| | More reference expression data |
|
|---|
| BioGPS | 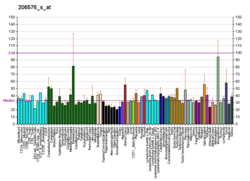

 | | More reference expression data |
|
|---|
|
| Gene ontology |
|---|
| Molecular function | - protein binding
- kinase binding
- protein homodimerization activity
- protein phosphatase binding
- protein tyrosine kinase binding
- filamin binding
- bile acid transmembrane transporter activity
- protein dimerization activity
- actin binding
- calmodulin binding
- molecular function
- identical protein binding
| | Cellular component | - membrane
- integral component of membrane
- extracellular region
- integral component of plasma membrane
- extracellular exosome
- T cell receptor complex
- cell-cell junction
- cell surface
- plasma membrane
- adherens junction
- basal plasma membrane
- apical plasma membrane
- lateral plasma membrane
- cell junction
- transport vesicle membrane
- cytoplasmic vesicle
- microvillus membrane
- specific granule membrane
- cell projection
- tertiary granule membrane
| | Biological process | - integrin-mediated signaling pathway
- cell migration
- angiogenesis
- leukocyte migration
- insulin catabolic process
- positive regulation of vasculogenesis
- cellular response to insulin stimulus
- negative regulation of cytotoxic T cell degranulation
- regulation of ERK1 and ERK2 cascade
- regulation of homophilic cell adhesion
- negative regulation of vascular permeability
- regulation of endothelial cell migration
- negative regulation of interleukin-1 production
- homophilic cell adhesion via plasma membrane adhesion molecules
- granulocyte colony-stimulating factor signaling pathway
- wound healing, spreading of cells
- negative regulation of hepatocyte proliferation
- negative regulation of granulocyte differentiation
- negative regulation of platelet aggregation
- blood vessel development
- negative regulation of lipid biosynthetic process
- bile acid and bile salt transport
- regulation of blood vessel remodeling
- negative regulation of T cell receptor signaling pathway
- negative regulation of natural killer cell mediated cytotoxicity directed against tumor cell target
- regulation of cell growth
- insulin receptor internalization
- negative regulation of T cell mediated cytotoxicity
- regulation of sprouting angiogenesis
- negative regulation of protein kinase activity
- negative regulation of fatty acid biosynthetic process
- regulation of cell migration
- regulation of phosphatidylinositol 3-kinase signaling
- common myeloid progenitor cell proliferation
- regulation of epidermal growth factor receptor signaling pathway
- regulation of endothelial cell differentiation
- cell adhesion
- neutrophil degranulation
- cell-cell adhesion via plasma-membrane adhesion molecules
| | Sources:Amigo / QuickGO |
|
| Orthologs |
|---|
| Species | Human | Mouse |
|---|
| Entrez | | |
|---|
| Ensembl | | |
|---|
| UniProt | | |
|---|
| RefSeq (mRNA) | NM_001024912
NM_001184813
NM_001184815
NM_001184816
NM_001205344
|
|---|
NM_001712 |
| |
|---|
NM_001113368
NM_001113369
NM_007543 |
|
|---|
| RefSeq (protein) | NP_001020083.1
NP_001020083
NP_001171742
NP_001171744
NP_001171745
|
|---|
NP_001192273
NP_001703 |
| |
|---|
NP_001106839
NP_001106840
NP_031569 |
|
|---|
| Location (UCSC) | Chr 19: 42.51 – 42.56 Mb | Chr 7: 25.22 – 25.24 Mb |
|---|
| PubMed search | [3] | [4] |
|---|
|
| Wikidata |
| View/Edit Human | View/Edit Mouse |
|
Carcinoembryonic antigen-related cell adhesion molecule 1 (biliary glycoprotein) (CEACAM1) also known as CD66a (Cluster of Differentiation 66a), is a human glycoprotein, and a member of the carcinoembryonic antigen (CEA) gene family.[5]
Function
This gene encodes a member of the carcinoembryonic antigen (CEA) gene family, which belongs to the immunoglobulin superfamily. Two subgroups of the CEA family, the CEA cell adhesion molecules and the pregnancy-specific glycoproteins, are located within a 1.2 Mb cluster on the long arm of chromosome 19. Eleven pseudogenes of the CEA cell adhesion molecule subgroup are also found in the cluster. The encoded protein was originally described in bile ducts of liver as biliary glycoprotein. Subsequently, it was found to be a cell–cell adhesion molecule detected on leukocytes, epithelia, and endothelia. The encoded protein mediates cell adhesion via homophilic as well as heterophilic binding to other proteins of the subgroup. Multiple cellular activities have been attributed to the encoded protein, including roles in the differentiation and arrangement of tissue three-dimensional structure, angiogenesis, apoptosis, tumor suppression, metastasis, and the modulation of innate and adaptive immune responses. Multiple transcript variants encoding different isoforms have been reported, but the full-length nature of only two has been determined.[5]
In melanocytic cells CEACAM1 gene expression may be regulated by MITF.[6]
Interactions
CEACAM1 has been shown to interact with PTPN11[7] and Annexin A2.[8]
See also
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000079385 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000054385 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b "Entrez Gene: CEACAM1 carcinoembryonic antigen-related cell adhesion molecule 1 (biliary glycoprotein)".
- ^ Hoek KS, Schlegel NC, Eichhoff OM, Widmer DS, Praetorius C, Einarsson SO, Valgeirsdottir S, Bergsteinsdottir K, Schepsky A, Dummer R, Steingrimsson E (December 2008). "Novel MITF targets identified using a two-step DNA microarray strategy". Pigment Cell & Melanoma Research. 21 (6): 665–76. doi:10.1111/j.1755-148X.2008.00505.x. PMID 19067971. S2CID 24698373.
- ^ Huber M, Izzi L, Grondin P, Houde C, Kunath T, Veillette A, Beauchemin N (January 1999). "The carboxyl-terminal region of biliary glycoprotein controls its tyrosine phosphorylation and association with protein-tyrosine phosphatases SHP-1 and SHP-2 in epithelial cells". The Journal of Biological Chemistry. 274 (1): 335–44. doi:10.1074/jbc.274.1.335. PMID 9867848.
- ^ Kirshner J, Schumann D, Shively JE (December 2003). "CEACAM1, a cell-cell adhesion molecule, directly associates with annexin II in a three-dimensional model of mammary morphogenesis". The Journal of Biological Chemistry. 278 (50): 50338–45. doi:10.1074/jbc.M309115200. PMID 14522961.
Further reading
- Gray-Owen SD, Blumberg RS (June 2006). "CEACAM1: contact-dependent control of immunity". Nature Reviews. Immunology. 6 (6): 433–46. doi:10.1038/nri1864. PMID 16724098. S2CID 34156579.
- Svenberg T, Hammarström S, Zeromski J (June 1979). "Immunofluorescence studies on the occurrence and localization of the CEA-related biliary glycoprotein I (BGP I) in normal human gastrointestinal tissues". Clinical and Experimental Immunology. 36 (3): 436–41. PMC 1537745. PMID 385181.
- Thompson J, Zimmermann W, Osthus-Bugat P, Schleussner C, Eades-Perner AM, Barnert S, Von Kleist S, Willcocks T, Craig I, Tynan K (April 1992). "Long-range chromosomal mapping of the carcinoembryonic antigen (CEA) gene family cluster". Genomics. 12 (4): 761–72. doi:10.1016/0888-7543(92)90307-E. PMID 1572649.
- Kuroki M, Arakawa F, Matsuo Y, Oikawa S, Nakazato H, Matsuoka Y (April 1991). "Three novel molecular forms of biliary glycoprotein deduced from cDNA clones from a human leukocyte library". Biochemical and Biophysical Research Communications. 176 (2): 578–85. doi:10.1016/S0006-291X(05)80223-2. PMID 2025273.
- Hinoda Y, Neumaier M, Hefta SA, Drzeniek Z, Wagener C, Shively L, Hefta LJ, Shively JE, Paxton RJ (September 1988). "Molecular cloning of a cDNA coding biliary glycoprotein I: primary structure of a glycoprotein immunologically crossreactive with carcinoembryonic antigen". Proceedings of the National Academy of Sciences of the United States of America. 85 (18): 6959–63. Bibcode:1988PNAS...85.6959H. doi:10.1073/pnas.85.18.6959. PMC 282098. PMID 2457922.
- Barnett TR, Kretschmer A, Austen DA, Goebel SJ, Hart JT, Elting JJ, Kamarck ME (February 1989). "Carcinoembryonic antigens: alternative splicing accounts for the multiple mRNAs that code for novel members of the carcinoembryonic antigen family". The Journal of Cell Biology. 108 (2): 267–76. doi:10.1083/jcb.108.2.267. PMC 2115422. PMID 2537311.
- Neumaier M, Paululat S, Chan A, Matthaes P, Wagener C (November 1993). "Biliary glycoprotein, a potential human cell adhesion molecule, is down-regulated in colorectal carcinomas". Proceedings of the National Academy of Sciences of the United States of America. 90 (22): 10744–8. Bibcode:1993PNAS...9010744N. doi:10.1073/pnas.90.22.10744. PMC 47854. PMID 7504281.
- Formisano P, Najjar SM, Gross CN, Philippe N, Oriente F, Kern-Buell CL, Accili D, Gorden P (October 1995). "Receptor-mediated internalization of insulin. Potential role of pp120/HA4, a substrate of the insulin receptor kinase". The Journal of Biological Chemistry. 270 (41): 24073–7. doi:10.1074/jbc.270.26.15844. PMID 7592607.
- Frängsmyr L, Baranov V, Prall F, Yeung MM, Wagener C, Hammarström S (July 1995). "Cell- and region-specific expression of biliary glycoprotein and its messenger RNA in normal human colonic mucosa". Cancer Research. 55 (14): 2963–7. PMID 7606710.
- Najjar SM, Philippe N, Suzuki Y, Ignacio GA, Formisano P, Accili D, Taylor SI (July 1995). "Insulin-stimulated phosphorylation of recombinant pp120/HA4, an endogenous substrate of the insulin receptor tyrosine kinase". Biochemistry. 34 (29): 9341–9. doi:10.1021/bi00029a009. PMID 7626603.
- Nédellec P, Turbide C, Beauchemin N (July 1995). "Characterization and transcriptional activity of the mouse biliary glycoprotein 1 gene, a carcinoembryonic antigen-related gene". European Journal of Biochemistry. 231 (1): 104–14. doi:10.1111/j.1432-1033.1995.tb20676.x. PMID 7628460.
- Watt SM, Fawcett J, Murdoch SJ, Teixeira AM, Gschmeissner SE, Hajibagheri NM, Simmons DL (July 1994). "CD66 identifies the biliary glycoprotein (BGP) adhesion molecule: cloning, expression, and adhesion functions of the BGPc splice variant". Blood. 84 (1): 200–10. doi:10.1182/blood.V84.1.200.200. PMID 8018919.
- Hauck W, Nédellec P, Turbide C, Stanners CP, Barnett TR, Beauchemin N (July 1994). "Transcriptional control of the human biliary glycoprotein gene, a CEA gene family member down-regulated in colorectal carcinomas". European Journal of Biochemistry. 223 (2): 529–41. doi:10.1111/j.1432-1033.1994.tb19022.x. PMID 8055923.
- Barnett TR, Drake L, Pickle W (February 1993). "Human biliary glycoprotein gene: characterization of a family of novel alternatively spliced RNAs and their expressed proteins". Molecular and Cellular Biology. 13 (2): 1273–82. doi:10.1128/mcb.13.2.1273. PMC 359012. PMID 8423792.
- Kuroki M, Yamanaka T, Matsuo Y, Oikawa S, Nakazato H, Matsuoka Y (August 1995). "Immunochemical analysis of carcinoembryonic antigen (CEA)-related antigens differentially localized in intracellular granules of human neutrophils". Immunological Investigations. 24 (5): 829–43. doi:10.3109/08820139509060710. PMID 8543346.
- Huber M, Izzi L, Grondin P, Houde C, Kunath T, Veillette A, Beauchemin N (January 1999). "The carboxyl-terminal region of biliary glycoprotein controls its tyrosine phosphorylation and association with protein-tyrosine phosphatases SHP-1 and SHP-2 in epithelial cells". The Journal of Biological Chemistry. 274 (1): 335–44. doi:10.1074/jbc.274.1.335. PMID 9867848.
- Feuk-Lagerstedt E, Jordan ET, Leffler H, Dahlgren C, Karlsson A (November 1999). "Identification of CD66a and CD66b as the major galectin-3 receptor candidates in human neutrophils". Journal of Immunology. 163 (10): 5592–8. doi:10.4049/jimmunol.163.10.5592. PMID 10553088.
- Soni P, Lakkis M, Poy MN, Fernström MA, Najjar SM (June 2000). "The differential effects of pp120 (Ceacam 1) on the mitogenic action of insulin and insulin-like growth factor 1 are regulated by the nonconserved tyrosine 1316 in the insulin receptor". Molecular and Cellular Biology. 20 (11): 3896–905. doi:10.1128/MCB.20.11.3896-3905.2000. PMC 85733. PMID 10805733.
- Ergün S, Kilik N, Ziegeler G, Hansen A, Nollau P, Götze J, Wurmbach JH, Horst A, Weil J, Fernando M, Wagener C (February 2000). "CEA-related cell adhesion molecule 1: a potent angiogenic factor and a major effector of vascular endothelial growth factor". Molecular Cell. 5 (2): 311–20. doi:10.1016/S1097-2765(00)80426-8. PMID 10882072.
- Wang L, Lin SH, Wu WG, Kemp BL, Walsh GL, Hong WK, Mao L (August 2000). "C-CAM1, a candidate tumor suppressor gene, is abnormally expressed in primary lung cancers". Clinical Cancer Research. 6 (8): 2988–93. PMID 10955775.
External links
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
|
|---|
| 1–50 | |
|---|
| 51–100 | |
|---|
| 101–150 | |
|---|
| 151–200 | |
|---|
| 201–250 | |
|---|
| 251–300 | |
|---|
| 301–350 | |
|---|
 2gk2: Crystal structure of the N terminal domain of human CEACAM1
2gk2: Crystal structure of the N terminal domain of human CEACAM1



















